- Solutions
- Real World Evidence
- IMID Observatory
Join our IMID Observatory for immune mediated inflammatory diseases
The IMID Observatory offers hospitals valuable insights into the real-world impact of advanced systemic therapies for immune-mediated inflammatory diseases (IMIDs). Our insights help improve patient care, optimise treatment pathways, and contribute to data-driven advancements in IMID management.
Gain Unique Pan-European Insights
Evaluate Real-World Clinical Practice with Ongoing Patient Data
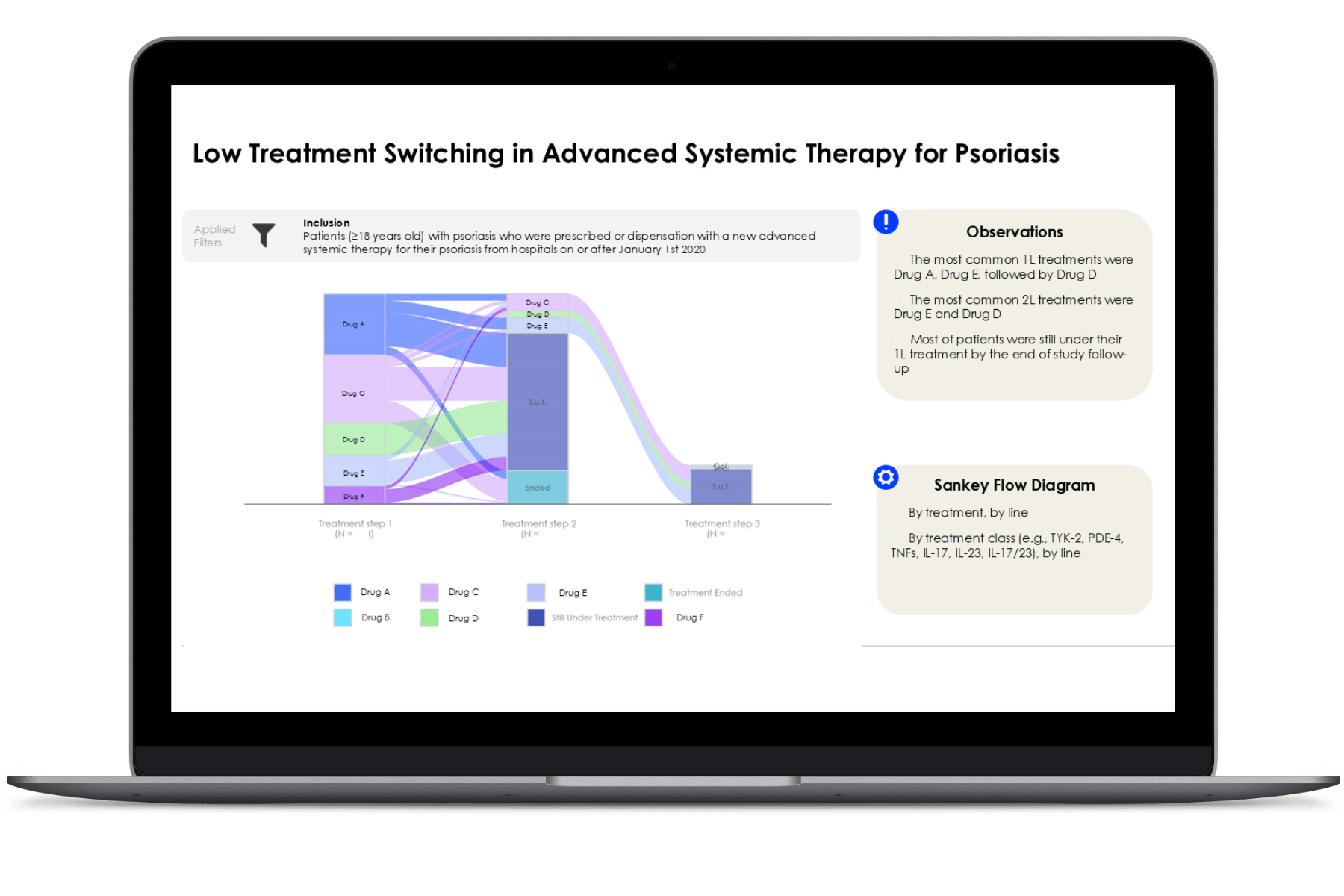
The IMID Observatory provides a unique opportunity to assess clinical practice through continuously updated data as patients move through various departments, including outpatient clinics and pharmacies. This dynamic information offers a comprehensive view of treatment pathways and patient outcomes, highlighting how therapies are applied in real-world settings. The ability to track these longitudinal data points across multiple touchpoints enables a deeper understanding of treatment effects, adherence to clinical guidelines, and opportunities for improvement, helping to refine strategies and optimise healthcare practices.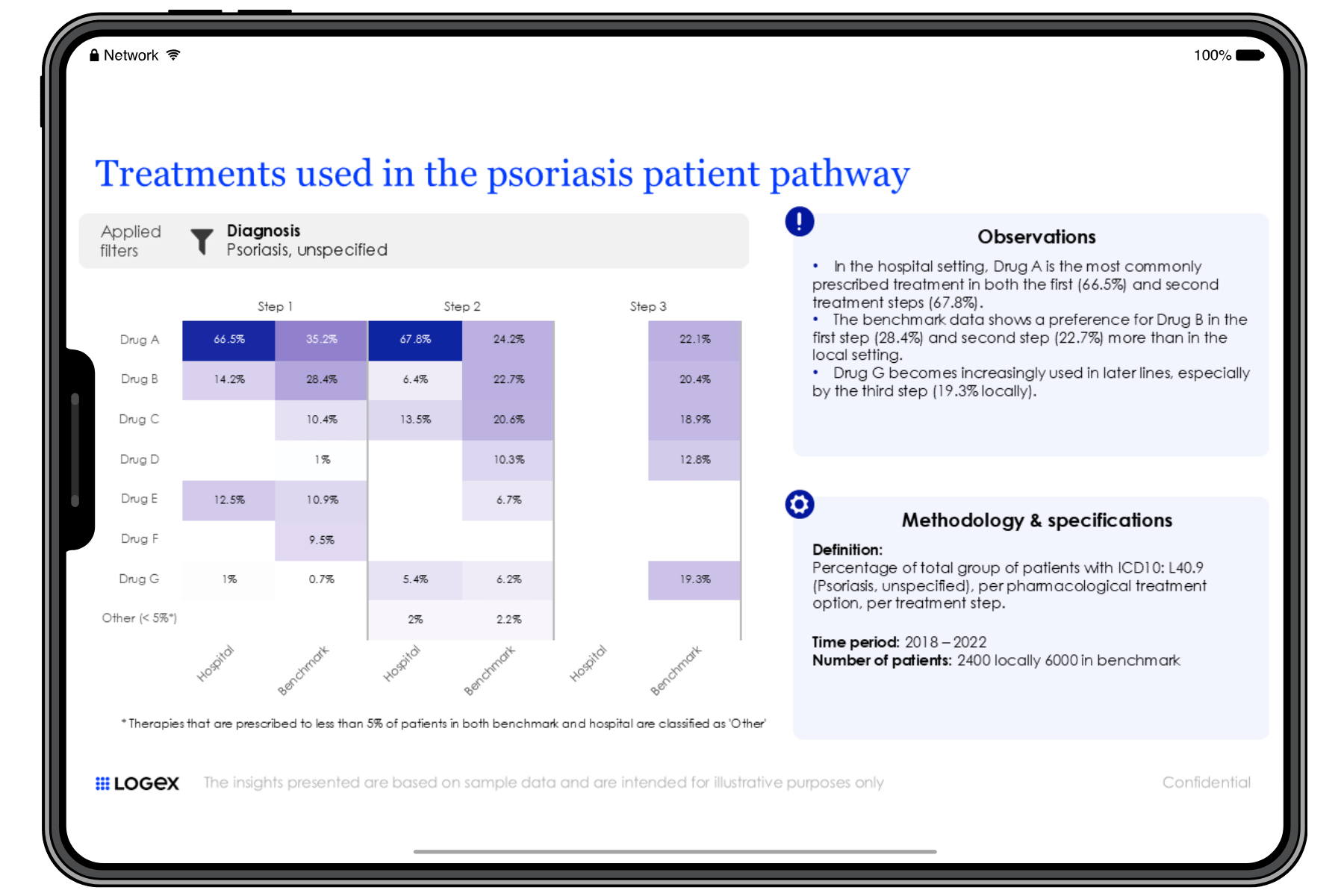
Maximising Hospital Efficiency and Patient Care
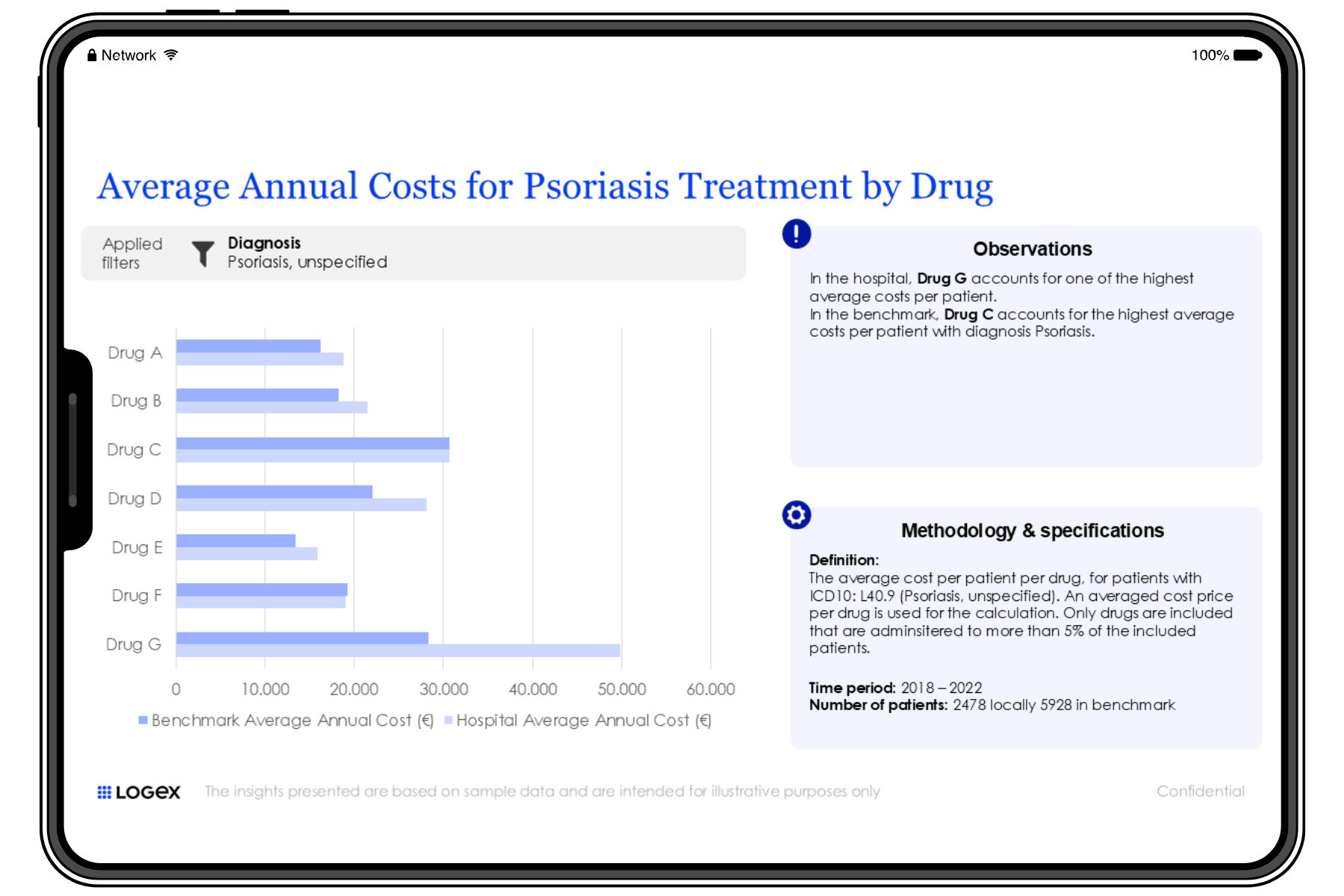
The IMID Observatory provides hospitals with invaluable insights into optimising patient care, improving patient flow, and managing healthcare resource utilisation (HCRU). By integrating data from various departments and clinical touchpoints, hospitals can refine their care pathways and treatment strategies. Leading clinicians and an expert advisory board are actively involved, ensuring that the insights generated are directly aligned with the real-world needs of hospitals. With a focus on enhancing health economics and clinical outcomes research (HEOR), the Observatory offers hospitals a comprehensive approach to improving clinical decision-making and operational efficiency.
Seamless Participation for Hospitals
Joining the IMID Observatory is simple and completely free of charge for hospitals. With minimal effort, hospitals can contribute valuable real-world data to a broader initiative that helps close knowledge gaps in the understanding of immunological diseases. By participating, hospitals not only benefit from actionable insights to improve patient care and optimise hospital interventions but also play a vital role in advancing healthcare knowledge. Your participation helps drive innovation and progress in the fight against complex conditions, contributing to the future of healthcare delivery.
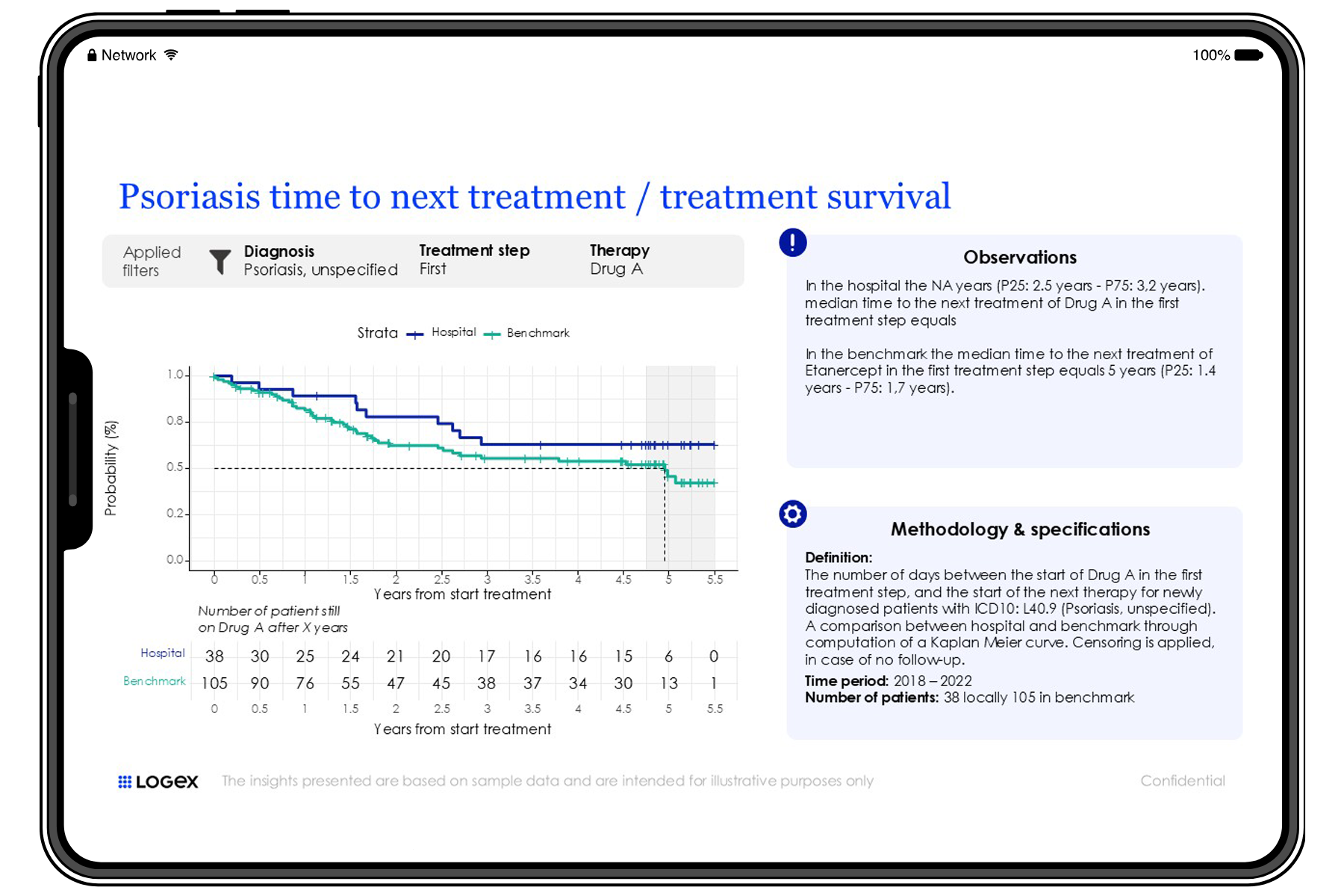
What makes our Observatory approach unique?

Data granularity
The level of detail LOGEX has access to is unique, allowing exceptional visibility into patient pathways. E.g.:
- Patient pathway mapping
- Lines of treatment
- Combinations of drugs and indication split
- Filter on specific patient groups

Data access & recency

Longitudinal insights

Scalability
The observatory setup allows for quick expansion into related disease areas under the same contracts.
With our extensive network of hospitals across Europe, expanding into other countries is highly feasible.

Experience
LOGEX has over 10 years of experience working with 700+ healthcare providers across the EU.
We are experts in extracting, delivering, validating and analysing healthcare datasets in close collaboration with HCPs.

Security by design
NEN7510 and ISO27001 certified, full compliance with GDPR.
FG and security officers, authorisation levels, two-factor authentication, informed / explicit consent.
Frequently Asked Questions
What is the legal basis for processing data in the Observatories?
The legal basis for processing data in our projects is provided by GDPR article 9.2(h), which covers projects designed to improve "the management of health or social care systems and services."
Who is responsible for data management, and how is it structured?
The hospital is the data controller, while LOGEX acts as the data processor, ensuring data is handled according to the legal and ethical guidelines.
Are there any specific requirements or ethical approvals needed for the Observatories?
Ethical approval might be necessary in specific cases and is dependent on local guidance and regulations. The focus of the observatory is to improve healthcare and outcomes for participating hospitals, which means ethical approval is not required in certain countries or specific situations.
How is hospital data handled and are there any restrictions on sharing it?
Hospitals remain the owners of their data at all times. Only anonymous population data may be shared with third parties, and LOGEX strictly follows local regulations regarding cross-border data transfers.
How does the IMID Observatory differ from traditional clinical registries, and what makes it particularly valuable?
Traditional clinical registries often focus on a single disease within a specific population, often limited by geographic or national boundaries. While they provide valuable insights, they may not capture the full complexity of multi-disease conditions like IMIDs, where patients often experience multiple related conditions. The IMID Observatory, by contrast, gathers continuous, real-world data from a broad network of hospitals across different countries, offering a more comprehensive view of patient care. This dynamic, international data collection allows for a deeper understanding of how different IMIDs, such as psoriasis and psoriatic arthritis, may intersect in real-world clinical settings.
Why do some patients with psoriasis later develop psoriatic arthritis?
Psoriasis and psoriatic arthritis (PsA) are both immune-mediated diseases, and there's a well-established link between the two. While psoriasis primarily affects the skin, in some patients, it can also progress to PsA, a condition where inflammation affects the joints. This is thought to occur due to shared underlying immune system mechanisms that drive both diseases. Around 30% of people with psoriasis eventually develop PsA, often starting with skin symptoms before joint pain appears. Understanding this progression is crucial for early detection and management, helping to improve patient outcomes by addressing both the skin and joint components of the disease.
How does the IMID Observatory help deepen our understanding of this connection?
The IMID Observatory plays a pivotal role in expanding knowledge about the progression from psoriasis to psoriatic arthritis. By continuously tracking patient data across different treatment stages and departments, the Observatory provides valuable insights into how psoriasis patients develop PsA and how different treatment strategies impact both skin and joint outcomes. This dynamic, real-time data allows clinicians and researchers to better understand patient pathways and the effect of therapies, helping close knowledge gaps and improve treatment strategies for those at risk of developing PsA.
How does this approach help with diseases like psoriasis that have multiple treatment options?
Psoriasis alone has in the EU over 16 different advanced systemic treatment options available, making it challenging to determine the most effective care pathways. The IMID Observatory tracks patient care not only for individual diseases but also how treatments impact patients with multiple IMIDs. This broader, patient-centered approach helps to uncover more nuanced insights into treatment effects, such as the sequencing of therapies and their impact on overall outcomes. By providing real-time, international data, the Observatory offers a more dynamic and in-depth perspective on care pathways compared to traditional, single-disease registries.
Get more out of our Healthcare Intelligence
Go beyond Real-World Data with Patient Engagement and Financial Analytics.

Financial Analytics
Controlling costs and optimising operations through data-driven decision-making.
Secure, compliant and trusted.
Healthcare organisations worldwide trust LOGEX.
Breaking Barriers in International Psoriasis Research Through Data Standardisation
Discover how LOGEX is transforming international psoriasis research through innovative data standardisation, enabling faster, more reliable insights across Europe.
Actionable insights into innovative medicines and their costs
Report: "On the Horizon".
March 2024 edition.

Learn how to leverage data
to make better decisions.
Read our latest publications about how we support data-driven decision-making.

The Hidden Connections Across Immune-Mediated Inflammatory Diseases: Why Collaborative Research Matters

AstraZeneca: Leveraging Real-World Data to Close Evidence Gaps in Clinical Practice
-1.jpg?width=1732&height=1155&name=Stocksy_txp3c7f9c9dMmT300_Medium_2944384-edit%20(1)-1.jpg)
How can RWE be used to solve unwarranted variation in drug uptake and clinical guidelines?
Get clarity and control to make better decisions.
Do you have any questions about harnessing data-driven insights for better decision-making? Allow our experts, such as Sarah, to lead the way.
We will reply within two business days.